Japan-based Olympus Corporation Acquires Israel-based Medi-Tate
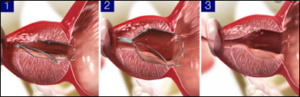
Adds an in-office treatment for benign prostatic hyperplasia (BPH) treatment to Olympus’ leading urological devices portfolio. Medi-Tate's “iTind” has received FDA de Novo authorization and a European CE mark. Since November 2018, Olympus secured distribution rights to iTind via an initial investment. The incidence of BPH is one of the most common diseases in aging men and the most common cause of lower urinary tract symptoms. According to the American Urological Association, BPH is a condition that impacts nearly 80% of males throughout their lifetime – with the ratio being higher in older ages. iTind is a temporarily implanted nitinol device, where the device is placed in the prostate in a folded configuration that is then gradually expanded to exert a gentle pressure at three precise points to reshape the prostatic urethra and bladder neck. After about a week, the device is completely removed, leaving a wider opening through which urine can flow for the relief of BPH symptoms. Terms not announced.
Keywords: Healthcare Investment Banking, Healthcare M&A, Healthcare Mergers, Medical Devices, Urology